In vitro fertilization (IVF) and other “high tech” procedures are now referred to as the assisted reproductive technologies (ART). These procedures all involve collecting the oocytes (eggs) and placing them in direct contact with sperm. Together they form an alphabet soup of techniques including: IVF, GIFT, ZIFT, ICSI, FET.
In its simplest term, IVF is simply the uniting of egg and sperm in vitro (in the lab). Subsequently the embryos are transferred into the uterus through the cervix and pregnancy is allowed to begin. IVF was the first of the ART techniques to be developed. The first birth was in 1978 in England. The procedure was pioneered by a Gynecologist and a Ph.D. ( Drs. Steptoe and Edwards). Next came GIFT which stands for gamete (egg and sperm) intrafallopian transfer. This procedure requires laparoscopy which is small incision surgery and requires a general anesthetic. With existing technology, pregnancy rates are similar with IVF and GIFT. Since IVF does not require surgery, it has supplanted GIFT.
ZIFT (zygote intrafallopian transfer) involves IVF and then a laparoscopic surgical procedure to transfer the embryos into the fallopian tube. Since transferring embryos through the cervix with IVF gives the same pregnancy rate as ZIFT, and is nonsurgical, IVF has also supplanted ZIFT.
As the years have passed, IVF has become the dominant ART technology due to its simplicity, efficacy and lack of invasiveness. A typical IVF cycle begins with shutting down the ovaries. This is done with a medication known as a GnRH agonist or a birth control pill. The most common drug that is used is Lupron. Lupron is given for approximately two weeks after which the ovaries are shut down temporarily. The reason for ovarian shut down is so that the follicles when stimulated will all ripen at approximately the same time. The next phase involves stimulation of the ovaries with potent ovulation medications such as Pergonal, Bravelle, Repronex, Gonal-f or Follistim. These are basically potent ovulation medications given to stimulate the development of several eggs. For a full description of these agents go to the page on ovulation medication. These injections are given for approximately 10 days. When the eggs are ready for harvesting, a final step is to give hCG to induce final maturation. The eggs are then harvested by a process called ultrasound guided vaginal retrieval. Under moderate sedation, and with ultrasound guidance, a thin needle is passed a short distance into the ovaries and the eggs are suctioned from the follicles. Typically 5-15 eggs are collected. Typically the eggs are fertilized by adding approximately 100,000 motile sperm to each egg. If the sperm will not fertilize the eggs naturally we can perform intracytoplasmic sperm injection (ICSI). This procedure involves injecting one sperm directly into the egg.
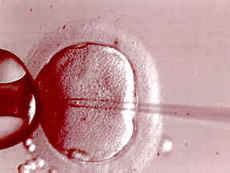
The day following retrieval, we can document fertilization under the microscope. We then observe the embryos for 3-6 days. Typically 1-2 embryos are then placed in a catheter and transferred through the cervix into the uterus 3 days following egg retrieval. The number of embryos to be transferred depends on the age of the woman. Women in the twenties have fewer and women in the thirties have more embryos transferred. An embryo transfer is a simple procedure much like a pap smear. At the present time, embryos can be transferred either 3 or five days following retrieval. A 3 day embryo is usually at the 6-8 cell stage.
For some photos of the IVF retrieval area and the embryo lab please go to IVF photo gallery.
It is also possible now in some cases to perform advanced stage or blastocyst embryo transfers.
Blastocyst transfer is appealing because of its ability to decrease multiple pregnancy rates. It does have its drawbacks. It can only be attempted if there are a high number of rapidly dividing embryos. This is because the majority of embryos cannot with existing technology make it to this final stage.
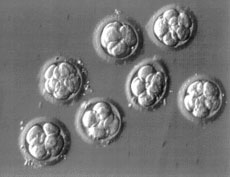
We have been working with blastocyst transfer for greater than 10 years and have been able to achieve excellent results with both fresh and frozen blastocysts. In March of 1999 channel 9 aired a story about 2 tristate couples. One had conceived with fresh and the other with frozen blastocysts. We find the technique to be helpful for many couples. All patients at the present time are considered for blastocyst transfer if they have 4 or more high quality embryos at 72 hours. At the present time, approximately 50% of our transfers are advance blastocyst transfers. Almost all of our embryo cryopreservation (freezing) is done at the blastocyst stage.
Two weeks after embryo transfer a pregnancy test can be obtained. Two weeks after the pregnancy test, an ultrasound can be performed and the fetal heart beat can be seen.
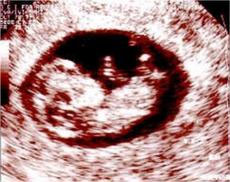
The image on the top is of a single pregnancy 6 weeks after egg retrieval and the one on the bottom is of a twin pregnancy. If more embryos were generated than can be replaced, these additional embryos can be saved by freezing (cryopreservation). Frozen embryos can be stored for future replacement at much lower cost than the original IVF cycle.
As the years have passed, IVF has improved greatly. Today it is arguably the most effective technique to treat infertility when compared with others on a month by month basis. IVF is not a perfect technology. First ,it is expensive. It may not work on the first cycle. Multiple pregnancies can result. The truth is that it is a powerful technology and must be used carefully. Some patients may have very high odds of success: 50 – 55% chance per attempt. Others may, due to their situation, have only a 20% chance of success. The age of the female partner is the single most important variable controlling pregnancy rates. Women in the twenties and early thirties have the best chance of success. The multiple pregnancy risk varies from 20% – 35% depending on clinic practices. Most multiples contrary to popular opinion are twins. Younger patients need fewer embryos to be replaced, and older patients need more. The worst thing that has happened with IVF is the various centers entering into a race to see who can get “the best statistics”. This has encouraged centers to transfer high numbers of embryos to get the stats while accepting too high a risk of multiple pregnancy. Also in order to get the best stats, some patients will be refused care in order to ” protect the statistics”.
Our data is contributed in great detail annually to the Center for Disease Control (CDC). The 2010 data have just been released. To see this information please click on the following CDC link: ivf data.
Date reporting typicall lags by 1 to 2 years to allow time for delivery of a given pregnancy. Our most recent review of our data from Oct 2011 to March 2012 showed the following clinical pregnancy rate per egg retrieval (52%). A clinical pregnancy is defined as a pregnancy that can be seen on ultrasound. Most such pregnancies will continue on to a live birth but some will miscarry.
The age < 37 Clinical pregnancy rate was 52%. Age of the egg is a major factor in predicting pregnancy rates. It is known that rates will decline with age. Another interesting statistic is our IVF money back program. This program is available for selected individuals lees than or equal to 37 years of age. Our live birth rate for this program is 80%. Money back program allows multiple cycles for a flat fee.
Our center was the first in the tristate area to report the birth of 1,000 IVF babies as of June of 1998. As of early 2012 we have now experienced more than 7,000 births. We were able to achieve these landmarks without refusing treatment to patients who were less likely to succeed. Our center has been successful with a variety of techniques including IVF, GIFT, frozen embryos, and egg donation. We have also been pleased with the ability of ICSI to help patients with very few sperm become parents. ICSI allows us to treat cases that were untreatable five years ago. We continue to improve the results of our program by participating in national and regional research trials. We also strive to improve clinical and laboratory techniques through implementation of promising new technologies.
In Vitro Fertilization and the assisted reproductive technologies (ART)
In Vitro Fertilization (IVF) was developed by a collaboration between Dr. Patrick Steptoe, a British gynecologist and Dr. Robert Edwards, a Ph.D. Dr. Steptoe was a pioneer in laparoscopy and had accumulated a group of patients who had absent fallopian tubes. Clearly the only way for them to conceive was to retrieve an egg, fertilize it in the lab, and then transfer the subsequent embryo into the uterus. Their early attempts were fraught with difficulties but none the less in 1978 they were successful with the delivery of Mary Louise Brown, “The World’s First Test Tube Baby”. Initially, In Vitro Fertilization was highly inefficient because of the use of the natural cycle and the ability to only retrieve one oocyte at a time. Also, the initial retrieval technique was by laparoscopy, which required a general anesthetic and a major surgical procedure to retrieve a single oocyte. By 1984, the first successful U. S. In Vitro Fertilization birth had occurred. From the mid 80’s to the early 90’s, the number of In Vitro Fertilization clinics increased dramatically in the United States. In the early 90’s this number was reduced as it became clear that it required considerable training and experience to operate a successful In Vitro Fertilization Program.
IVF is simply the uniting of egg and sperm in vitro, (i.e. in the laboratory). Subsequently, the embryo is transferred into the uterus and pregnancy is allowed to begin. Initially, this technique was available to women with severe tubal disease only. With increased experience with this technique, it was found that it was an effective treatment for almost all forms of intractable infertility. Therefore, individuals with endometriosis, ovulation problems, unexplained infertility, or male factor infertility could be treated with this technique if simpler measures were not effective.
The starting point in the procedure is called superovulation. Superovulation involves the recruitment of multiple follicles, which are the structures within which the egg develops. Modern techniques of superovulation include many different hormone and drug combinations. The most typically used stimulatory agents are potent injectable drugs, such as Humegon, Repronex, Gonal-F or Follistim. The most common pre-treatment is with a medication known as Lupron. Lupron pre-treatment tends to allow for more parallel development of follicles so on the day of harvest more mature oocytes are available for pick up. Also, Lupron pre-treatment decreases the cancellation rate of the cycle due to premature ovulatory signals. Because of this, a combination of Lupron pre-treatment followed by a potent injectable drug such as Gonal-F or Follistim is probably the most popular worldwide for preparation for In Vitro Fertilization. Typically, in the month prior to the one intended to be used for oocyte harvest, Lupron is begun. Lupron injections are given subcutaneously until there is evidence of shutdown of the ovaries. Following this several days of an injectable agent, such a Gonal-F, is initiated.
After 8 to 9 days of injections the follicles are usually considered mature and human chorionic gonadotropin (hCG) is administered. hCG is literally a trigger for ovulation. It allows for final maturation of the oocytes. Thirty-six hours after the hCG is given, and immediately prior to ovulation, oocyte retrieval is planned.
Oocyte retrieval can be accomplished by a variety of different techniques. At the present time the worldwide consensus is that transvaginal retrieval is the least traumatic way of performing the procedure. This type of technique is also called a nonsurgical ultrasound guided retrieval. The technique relies heavily on the normal pelvic anatomy. It is well known that the ovaries are in very close proximity to the upper vagina. Using transvaginal retrieval an ultrasound probe is placed in the upper vagina and is used to guide a narrow gauged needle into the follicles. Typically this is done under light anesthesia. The patient does not have to be “put to sleep”. An egg retrieval takes 30 minutes to complete. There is minimal or no discomfort associated with it. A typical number of eggs retrieved is 5-12. For some photos of the IVF retrieval area and embryo lab, please go to: IVF photo gallery.
Following egg retrieval the husband is asked to do a sperm collection. The sperm is processed by a technique known as “washing”. This is to remove any additional cellular material or chemical substances within the ejaculate. A pure suspension of sperm is then added to the eggs. Each egg is inseminated with 50,000-100,000 motile spermatozoa. If semen parameters are poor, direct Intracytoplasmic Sperm Injection (ICSI) is performed. ICSI has revolutionized IVF. We are now able to treat cases where only a few sperm are in the ejaculate. Also, we can collect sperm directly from the testes in cases where the sperm count is zero and use these for ICSI. ICSI has been used widely for 6 years in the U.S. Because ICSI involves the lab picking and injecting the sperm into the egg, the process of “natural selection” is eliminated. There were initial concerns that ICSI may increase the incidence of birth defects. The current information is that ICSI increases the incidence of birth defects by 0-1%. It will take more births to narrow the interval further. Most birth defects appear to be minor, correctable by plastic surgical procedures. IVF has been around since 1978 and we know that it does not increase the incidence of birth defects. Embryo freezing has been available since 1985 and it is known that freezing does not increase the incidence of birth defects. The overall incidence of birth defects in the general population is 3-5%.
The day following the retrieval we can examine the oocytes for evidence of fertilization. If there are an excess number of embryos, a decision can be made regarding cryopreservation or freezing. Generally speaking, in a woman under the age of 35, it would be considered appropriate to transfer up to 2-3 embryos. In women 35-40, each program generally individualizes based on their results. In our program we recommend the transfer of 3-5 embryos because we have noticed that the implantation rate is slightly lower. Transferring higher number of embryos would generally improve the pregnancy rate, but will also increase the multiple pregnancy rate.
Embryo transfer is much like a slowly conducted pelvic examination. A speculum is placed in the vagina. The vagina is cleansed with sponges. A premeasured catheter is then advanced to the very top of the uterus where the embryos are deposited in a very small droplet of fluid. Following embryo transfer, the patient will usually rest quietly for 30 minutes prior to her departure for home. The 30 minute rest interval is not thought to be important. Over the years we have decreased the rest interval from 6 hours to 30 minutes and have experienced increasing pregnancy rates. Following embryo transfer we supplement the ovaries’ production of progesterone with injectable progesterone in oil. This is continued for two weeks until a pregnancy test is obtained.
The success rate of the In Vitro Fertilization is highly variable among programs. Also it is now known that the age of the woman is the single most important variable controlling the success rate of IVF. For example, a woman in the twenties will have twice the pregnancy rate of a woman in the forties. The ideal way to analyze results is to look at the clinical pregnancy rate. A clinical pregnancy is one that can be seen with ultrasound. This excludes early or biochemical pregnancies, which are documented by blood testing but never get to the point of being seen with ultrasound. In a good program the pregnancy rate is in the range of 40-50% per egg retrieval. Each year the CDC publishes the data of all reporting clinics. The 2003 data has just been posted on the CDC web site. To view this data directly please use the following link: SART data. Multiple pregnancies are relatively common due to the transfer of more than one embryo. The multiple pregnancy rates will vary between 20-30%. The vast majority of multiple pregnancies are twin pregnancies. A few percent of multiple pregnancies will be triplet or, less commonly, quadruplet pregnancies. Multiple pregnancies are a great concern due to the risk of premature delivery. Prematurely- delivered babies may require assisted respiration and other medical intervention. Of all clinical pregnancies that are generated approximately 20% will be lost to miscarriages. Miscarriages are not any more common with high tech pregnancies than with natural conception. A few percent of all pregnancies generated by IVF will be tubal pregnancies. Over the years IVF has improved and become more predictable and useful as a clinical tool. The ideal candidate for the procedure is still someone with tubal disease where we can achieve our highest pregnancy rate. Many other individuals, however, can succeed with this procedure. The challenge in the future is to continually improve the pregnancy rate. When this occurs, IVF will become the dominant technology because it will be both efficient and simple.
If In Vitro Fertilization results in more embryos than can be transferred, freezing can be used to save the extra embryos. Therefore, in a young woman, if 6 embryos are generated, frequently 2 are transferred and 4 are frozen for future use. If the patient conceives with her fresh embryo transfer, the frozen embryos can later be used to generate a second pregnancy. If the patient does not conceive with the fresh embryo transfer then, after waiting 1 to 2 months, the frozen embryos can be transferred into the uterus to establish an initial pregnancy. The advantage of the availability of the frozen embryos is that frozen embryo transfer is much less costly and time consuming because it does not require a retrieval. One must simply wait one to two days after ovulation and then thaw and transfer the embryos. The pregnancy rate from a frozen embryo transfer is approximately 25%. While this is not as good as the fresh embryo pregnancy rate the amount of effort associated with a frozen embryo transfer is smaller. Embryo cryopreservation has been successful since the mid-80’s. The pregnancy rates have continued to improve over the years.
While In Vitro Fertilization came to us from the United Kingdom, GIFT is an American innovation. The first successful case of GIFT was reported by Dr. Ricardo Asch in 1984. GIFT refers to the transfer of gametes “eggs and sperm” into the fallopian tube. Gametes normally meet in the fallopian tube. It was hoped that in some ways that GIFT would be “natural” and would offer improved pregnancy rates. Stimulation protocols for GIFT are identical for IVF. In contradistinction to IVF, GIFT is done by laparoscopy and under a general anesthetic. This is done in order to access both the ovaries and the fallopian tubes. The procedure is done by aspirating all of the follicles in order to retrieve the maximum number of eggs. A plastic tube, “catheter” is then loaded with a certain number of oocytes, which can vary from 4 to 6 as well as 100,000 motile spermatozoa. One or both fallopian tubes are then elevated and cannulated. The sperm and eggs are deposited in the mid-portion of the fallopian tubes. The case is then finished and the eggs and sperm are then allowed to unite in the fallopian tube where natural/normal fertilization occurs. Possible advantages of GIFT include the fact that it is more “natural”. However, it is more important to understand that comparing GIFT to IVF is much like comparing apples and oranges. GIFT is more involved due to its requirement for laparoscopy, which is a major surgical procedure. Also, GIFT carries the disadvantage of not allowing us to observe fertilization. Therefore, if a couple does not conceive from GIFT one is uncertain whether this critical process has occurred. It is felt that in the hands of a good embryo laboratory, the results from GIFT and IVF will be fairly similar.
The assisted reproductive technologies are rapidly evolving. Every year there are new discoveries in this area. For instances, we now know that the presence of a hydrosalpinx, which is a tube dilated with fluid, is detrimental to IVF. Today we would remove the hydrosalpinx prior to proceeding with IVF. This was not the case 3 years ago. Also, we have a better understanding of the chemical environment necessary for optimal embryo development and this has been reflected in improvements in the culture media used.
We have also wanted to reduce the number of multiple pregnancies generated. This is now possible through the selection of a fewer number of higher quality embryos for transfer. This technique, called subselection, has improved pregnancy rates and lowered the number of multiple pregnancies generated.
Trying to determine which patient will benefit from IVF and which patient will not do well is very important. This technique is called screening for ovarian reserve. It tells us if the number of high quality eggs is diminished or normal. We can now do this with blood testing. We can check the Day 3 FSH level. We can also do, if necessary, a clomiphene challenge test.
Intracytoplasmic sperm injection (ICSI) has revolutionized IVF. We can today treat successfully cases that were previously impossible to treat. Pregnancies have been established in our program with as few as four sperm identified on the days of the IVF procedure. There are many cases where we simply could not obtain fertilization with standard IVF that are easily treated with ICSI.
The field of assisted reproductive technology has undergone tremendous change since the birth of Mary Louise Brown twenty years ago. The field continues to evolve and indeed we can make the claim that in ART, nothing is constant but change itself! The challenge for us is to use this technology as wisely as possible in each case. We have come a long way in the past two decades and hope to see even more progress in the decades to follow.